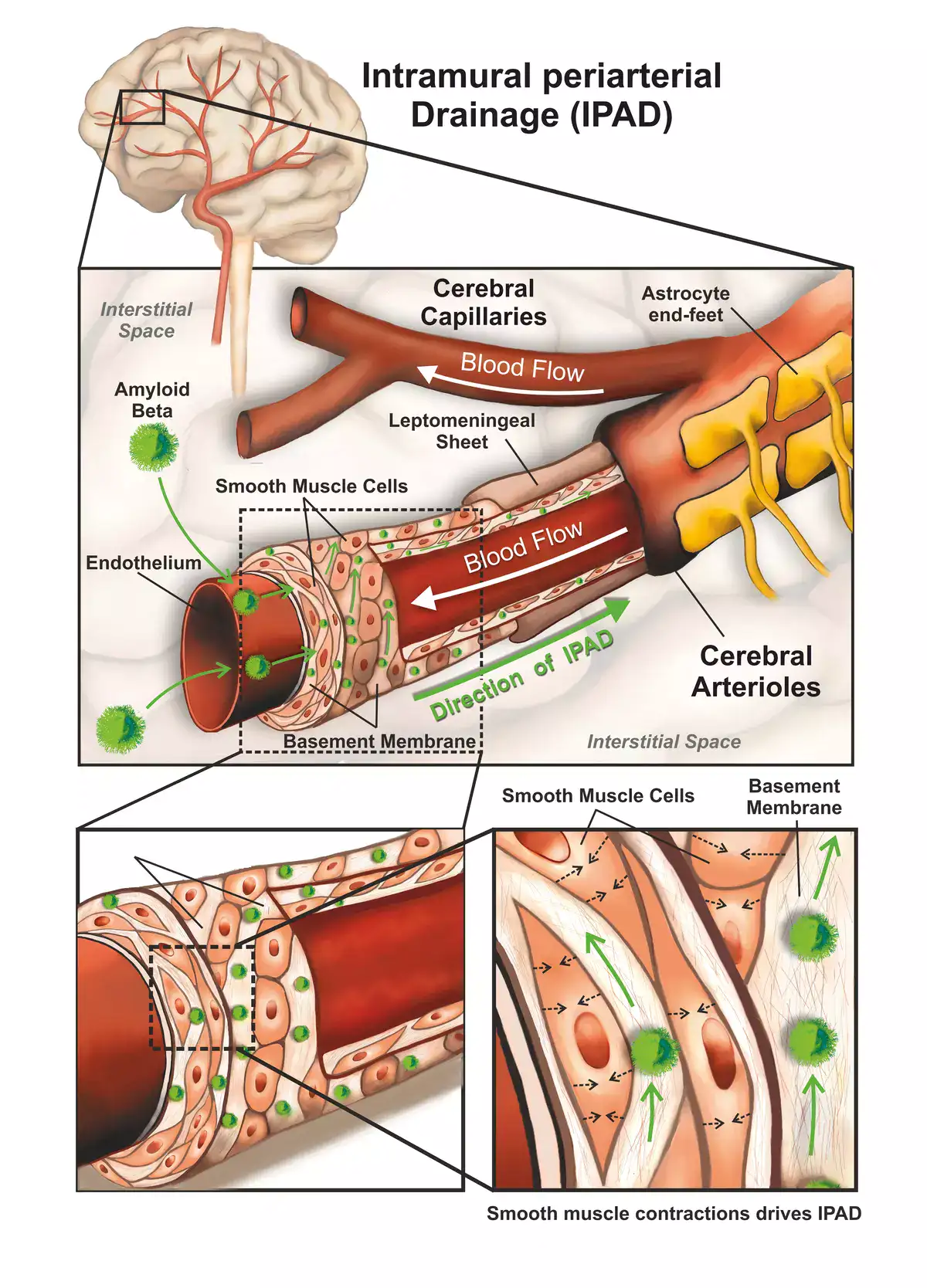
Defining an Ageing-Related Pathology, Disease or Syndrome: International Consensus Statement
Short E, Adcock IM, Al-Sarireh B, Ager A, Ajjan R, Akbar N, Akeroyd MA, Alsaleh G, Al-Sharbatee G, Alavian K, Amoaku W, Andersen J, Antoniades C, Arends MJ, Astley S, Atan D, Attanoos R, Attems J, Bain S, Balaskas K, Balmus G, Bance M, Barber TM, Bardhan A, Barker K, Barnes P, Basatemur G, Bateman A, Bauer ME, Bellamy C, van Beek E, Bellantuono I, Benbow E, Bhandari S, Bhatnagar R, Bloom P, Bowdish D, Bowerman M, Burke M, Carare R, Carrington EV, Castillo-Quan JI, Clegg P, Cole J, Cota C, Chazot P, Chen C, Cheong Y, Christopher G, Church G, Clancy D, Cool P, Del Galdo F, Dalakoti M, Dasgupta S, Deane C, Dhasmana D, Dojcinov S, Di Prete M, Du H, Duggal NA, Ellmers T, Emanueli C, Emberton M, Erusalimsky JD, Feldmeyer L, Fleming A, Forbes K, Foster TC, Frasca D, Frayling I, Freedman D, Fülöp T, Ellison-Hughes G, Gazzard G, George C, Gil J, Glassock R, Goldin R, Green J, Guymer R, Haboubi H, Harries L, Hart S, Hartley D, Hasaballa S, Henein C, Helliwell M, Henderson E, Heer R, Holte K, Idris I, Isenburg D, Jylhävä J, Iqbal A, Jones SW, Kalaria R, Kanamarlapudi V, Kempf W, Kermack AJ, Kerns J, Koulman A, Khan AH, Kinross J, Klaucane K, Krishna Y, Gill HS, Lakatta E, Laconi E, Lazar A, Leeuwenburgh C, Leung S, Li X, van der Linde I, Lopes LV, Lorenzini A, Lotery A, Machado P, Mackie S, Madeddu P, Maier A, Mukkanna K, Manousou P, Markey O, Mauro C, McDonnell B, Medina RJ, Meran S, Metzler-Baddeley C, Meglinksi I, Milman N, Mitteldorf C, Montgomery R, Morris AC, Mühleisen B, Mukherkee A, Murray A, Nelson S, Nicolaou A, Nirenberg A, Noble S, Nolan LS, Nus M, Van On C, Osei-Lah V, Peffers M, Palmer A, Palmer D, Palmer L, Parry-Smith W, Pawelec G, Peleg S, Perera R, Pitsillides A, Plack CJ, Progatzsky F, Pyott S, Rajput K, Rashid S, Ratnayaka JA, Ratnayake SAB, Rodriguez-Justo M, Rosa AC, Rule A, Sanger GJ, Sayers I, Saykin A, Selvarajah D, Sethi J, Shanahan C, Shen-Orr S, Sheridan C, Shiels P, Sidlauskas K, Sivaprasad S, Sluimer J, Small G, Smith P, Smith R, Snelling S, Spyridopoulos I, Srinivasa Raghavan R, Steel D, Steel KP, Stewart C, Stone K, Subbarayan S, Sussman M, Svensson J, Tadanki V, Tan AL, Tanzi RE, Tatler A, Tavares AAS, Tengku Mohd TAM, Tiganescu A, Timmons J, Tree J, Trivedi D, Tsochatzis EA, Tsimpida D, Vinke EJ, Whittaker A, Vallabh NA, Veighey K, Venables ZC, Reddy V, Vernooij MW, Verschoor C, Vinciguerra M, Vukanovic V, Vyazovskiy V, Walker J, Wakefield R, Watkins AJ, Webster A, Weight C, Weinberger B, Whitney SL, Willis R, Witkowski JM, Yeo LLL, Chung TY, Yu E, Zemel M, Calimport SRG and Bentley BL
Defining an Ageing-Related Pathology, Disease or Syndrome: International Consensus Statement
Short E, Adcock IM, Al-Sarireh B, Ager A, Ajjan R, Akbar N, Akeroyd MA, Alsaleh G, Al-Sharbatee G, Alavian K, Amoaku W, Andersen J, Antoniades C, Arends MJ, Astley S, Atan D, Attanoos R, Attems J, Bain S, Balaskas K, Balmus G, Bance M, Barber TM, Bardhan A, Barker K, Barnes P, Basatemur G, Bateman A, Bauer ME, Bellamy C, van Beek E, Bellantuono I, Benbow E, Bhandari S, Bhatnagar R, Bloom P, Bowdish D, Bowerman M, Burke M, Carare R, Carrington EV, Castillo-Quan JI, Clegg P, Cole J, Cota C, Chazot P, Chen C, Cheong Y, Christopher G, Church G, Clancy D, Cool P, Del Galdo F, Dalakoti M, Dasgupta S, Deane C, Dhasmana D, Dojcinov S, Di Prete M, Du H, Duggal NA, Ellmers T, Emanueli C, Emberton M, Erusalimsky JD, Feldmeyer L, Fleming A, Forbes K, Foster TC, Frasca D, Frayling I, Freedman D, Fülöp T, Ellison-Hughes G, Gazzard G, George C, Gil J, Glassock R, Goldin R, Green J, Guymer R, Haboubi H, Harries L, Hart S, Hartley D, Hasaballa S, Henein C, Helliwell M, Henderson E, Heer R, Holte K, Idris I, Isenburg D, Jylhävä J, Iqbal A, Jones SW, Kalaria R, Kanamarlapudi V, Kempf W, Kermack AJ, Kerns J, Koulman A, Khan AH, Kinross J, Klaucane K, Krishna Y, Gill HS, Lakatta E, Laconi E, Lazar A, Leeuwenburgh C, Leung S, Li X, van der Linde I, Lopes LV, Lorenzini A, Lotery A, Machado P, Mackie S, Madeddu P, Maier A, Mukkanna K, Manousou P, Markey O, Mauro C, McDonnell B, Medina RJ, Meran S, Metzler-Baddeley C, Meglinksi I, Milman N, Mitteldorf C, Montgomery R, Morris AC, Mühleisen B, Mukherkee A, Murray A, Nelson S, Nicolaou A, Nirenberg A, Noble S, Nolan LS, Nus M, Van On C, Osei-Lah V, Peffers M, Palmer A, Palmer D, Palmer L, Parry-Smith W, Pawelec G, Peleg S, Perera R, Pitsillides A, Plack CJ, Progatzsky F, Pyott S, Rajput K, Rashid S, Ratnayaka JA, Ratnayake SAB, Rodriguez-Justo M, Rosa AC, Rule A, Sanger GJ, Sayers I, Saykin A, Selvarajah D, Sethi J, Shanahan C, Shen-Orr S, Sheridan C, Shiels P, Sidlauskas K, Sivaprasad S, Sluimer J, Small G, Smith P, Smith R, Snelling S, Spyridopoulos I, Srinivasa Raghavan R, Steel D, Steel KP, Stewart C, Stone K, Subbarayan S, Sussman M, Svensson J, Tadanki V, Tan AL, Tanzi RE, Tatler A, Tavares AAS, Tengku Mohd TAM, Tiganescu A, Timmons J, Tree J, Trivedi D, Tsochatzis EA, Tsimpida D, Vinke EJ, Whittaker A, Vallabh NA, Veighey K, Venables ZC, Reddy V, Vernooij MW, Verschoor C, Vinciguerra M, Vukanovic V, Vyazovskiy V, Walker J, Wakefield R, Watkins AJ, Webster A, Weight C, Weinberger B, Whitney SL, Willis R, Witkowski JM, Yeo LLL, Chung TY, Yu E, Zemel M, Calimport SRG and Bentley BL
Around the world, individuals are living longer, but an increased average lifespan does not always equate to an increased healthspan. With advancing age, the increased prevalence of ageing-related diseases can have a significant impact on health status, functional capacity, and quality of life. It is therefore vital to develop comprehensive classification and staging systems for ageing-related pathologies, diseases and syndromes. This will allow societies to better identify, quantify, understand, and meet the healthcare, workforce, wellbeing, and socioeconomic needs of ageing populations, while supporting the development and utilisation of interventions to prevent or to slow, halt or reverse the progression of ageing-related pathologies.
Alzheimer's disease pathophysiology in the Retina
Gaire BP, Koronyo Y, Fuchs DT, Shi H, Rentsendorj A, Danziger R, Vit JP, Mirzaei N, Doustar J, Sheyn J, Hampel H, Vergallo A, Davis MR, Jallow O, Baldacci F, Verdooner SR, Barron E, Mirzaei M, Gupta VK, Graham SL, Tayebi M, Carare RO, Sadun AA, Miller CA, Dumitrascu OM, Lahiri S, Gao L, Black KL and Koronyo-Hamaoui M
Alzheimer's disease pathophysiology in the Retina
Gaire BP, Koronyo Y, Fuchs DT, Shi H, Rentsendorj A, Danziger R, Vit JP, Mirzaei N, Doustar J, Sheyn J, Hampel H, Vergallo A, Davis MR, Jallow O, Baldacci F, Verdooner SR, Barron E, Mirzaei M, Gupta VK, Graham SL, Tayebi M, Carare RO, Sadun AA, Miller CA, Dumitrascu OM, Lahiri S, Gao L, Black KL and Koronyo-Hamaoui M
The retina is an emerging CNS target for potential noninvasive diagnosis and tracking of Alzheimer's disease (AD). Studies have identified the pathological hallmarks of AD, including amyloid β-protein (Aβ) deposits and abnormal tau protein isoforms, in the retinas of AD patients and animal models. Moreover, structural and functional vascular abnormalities such as reduced blood flow, vascular Aβ deposition, and blood-retinal barrier damage, along with inflammation and neurodegeneration, have been described in retinas of patients with mild cognitive impairment and AD dementia. Histological, biochemical, and clinical studies have demonstrated that the nature and severity of AD pathologies in the retina and brain correspond. Proteomics analysis revealed a similar pattern of dysregulated proteins and biological pathways in the retina and brain of AD patients, with enhanced inflammatory and neurodegenerative processes, impaired oxidative-phosphorylation, and mitochondrial dysfunction. Notably, investigational imaging technologies can now detect AD-specific amyloid deposits, as well as vasculopathy and neurodegeneration in the retina of living AD patients, suggesting alterations at different disease stages and links to brain pathology. Current and exploratory ophthalmic imaging modalities, such as optical coherence tomography (OCT), OCT-angiography, confocal scanning laser ophthalmoscopy, and hyperspectral imaging, may offer promise in the clinical assessment of AD. However, further research is needed to deepen our understanding of AD's impact on the retina and its progression. To advance this field, future studies require replication in larger and diverse cohorts with confirmed AD biomarkers and standardized retinal imaging techniques. This will validate potential retinal biomarkers for AD, aiding in early screening and monitoring.
[1-C]-Butanol Positron Emission Tomography reveals an impaired brain to nasal turbinates pathway in aging amyloid positive subjects
Mehta NH, Wang X, Keil SA, Xi K, Zhou L, Lee K, Tan W, Spector E, Goldan A, Kelly J, Karakatsanis NA, Mozley PD, Nehmeh S, Chazen JL, Morin S, Babich J, Ivanidze J, Pahlajani S, Tanzi EB, Saint-Louis L, Butler T, Chen K, Rusinek H, Carare RO, Li Y, Chiang GC and de Leon MJ
[1-C]-Butanol Positron Emission Tomography reveals an impaired brain to nasal turbinates pathway in aging amyloid positive subjects
Mehta NH, Wang X, Keil SA, Xi K, Zhou L, Lee K, Tan W, Spector E, Goldan A, Kelly J, Karakatsanis NA, Mozley PD, Nehmeh S, Chazen JL, Morin S, Babich J, Ivanidze J, Pahlajani S, Tanzi EB, Saint-Louis L, Butler T, Chen K, Rusinek H, Carare RO, Li Y, Chiang GC and de Leon MJ
Reduced clearance of cerebrospinal fluid (CSF) has been suggested as a pathological feature of Alzheimer's disease (AD). With extensive documentation in non-human mammals and contradictory human neuroimaging data it remains unknown whether the nasal mucosa is a CSF drainage site in humans. Here, we used dynamic PET with [1-C]-Butanol, a highly permeable radiotracer with no appreciable brain binding, to test the hypothesis that tracer drainage from the nasal pathway reflects CSF drainage from brain. As a test of the hypothesis, we examined whether brain and nasal fluid drainage times were correlated and affected by brain amyloid.